Plain language summary of the CARTITUDE-4 study of cilta-cel or standard care in people with lenalidomide-refractory multiple myeloma
- Julia Cope
- Jul 12, 2024
- 7 min read
Updated: Nov 15, 2024
Disclosures
This summary was written by Julia Cope as a spec sample to demonstrate writing capabilities to prospective clients. It was developed using the Envision Pharma Group Plain Language Summaries of Publications Toolkit (CC BY 4.0). Neither the study sponsor nor the original authors of the full article were involved in preparing this summary.
What is the purpose of this summary?
The aim of this summary is to help people with multiple myeloma, their caregivers, and healthcare providers understand recent results from CARTITUDE-4, an ongoing clinical study comparing ciltacabtagene autoleucel (cilta-cel) with standard care treatment in people with multiple myeloma.
Based on results from CARTITUDE-4, cilta-cel was approved in the USA and Europe in April 2024 as a treatment for adults with relapsed or refractory multiple myeloma who have already received at least one line of treatment for their disease, including an immunomodulatory agent and a proteasome inhibitor, and whose disease doesn’t respond to lenalidomide. This summary is intended to help you understand the clinical trial results that led to cilta-cel’s expanded approval.

Some helpful definitions
What is relapsed or refractory multiple myeloma?
Multiple myeloma is a cancer of the bone marrow that develops in plasma cells, a type of white blood cell. There are medicines available to treat multiple myeloma. However, most people will find that their cancer eventually comes back (relapsed disease), or worsens even while treatment is being received (refractory disease).
What is lenalidomide-refractory multiple myeloma?
Lenalidomide is an anti-cancer medicine that is commonly used to treat people with multiple myeloma. It works by activating the immune system to kill cancer cells. People with lenalidomide-refractory multiple myeloma have cancer that doesn’t respond, or has stopped responding to, lenalidomide treatment. These people often have faster disease progression and a shorter life-expectancy than those who do respond to lenalidomide. Therefore, there is a need for new therapies to treat people with lenalidomide-refractory multiple myeloma.
What is cilta-cel?
People with multiple myeloma accumulate the cancerous multiple myeloma cells in their bone
marrow. Multiple myeloma cells have a protein on their surface called B-cell maturation antigen (BCMA). BCMA is found in higher amounts on multiple myeloma cells than on normal cells. Cilta-cel is a medicine that targets BCMA, made from a multiple myeloma patient’s own T-cells. To produce cilta-cel, T-cells are collected from the patient’s blood and genetically modified to make receptors, called chimeric antigen receptors (CARs), that recognize BCMA. T-cells with these new receptors are known as CAR-T cells. After being made, cilta-cel CAR-T cells are given back to the same patient in a single infusion, where they multiply and move throughout the body. Now, the cells that recognize BCMA can identify multiple myeloma cells and destroy them.
What did this phase 3 study look at?
Phase 3 studies are conducted to compare the safety and ability of a new medicine to treat a particular disease compared with current standard care treatments that are already known to be effective. In CARTITUDE-4, researchers compared cilta-cel treatment with standard care in people with lenalidomide-refractory multiple myeloma. In an earlier clinical study called CARTITUDE-1, cilta-cel reduced signs of multiple myeloma in people with who had already received 3 or more different lines of therapy (treatment approaches) for their disease. To see if cilta-cel can safely and effectively treat multiple myeloma if it is used earlier in the treatment course, CARTITUDE-4 looked at outcomes in people who had received either 1, 2, or 3 lines of therapy for their cancer. Specifically, they wanted to know:
How long participants live without their cancer getting worse.
How many participants respond to treatment by showing fewer signs of disease.
How much participants respond to treatment.
What side effects participants experience during treatment.
Who took part in the study?
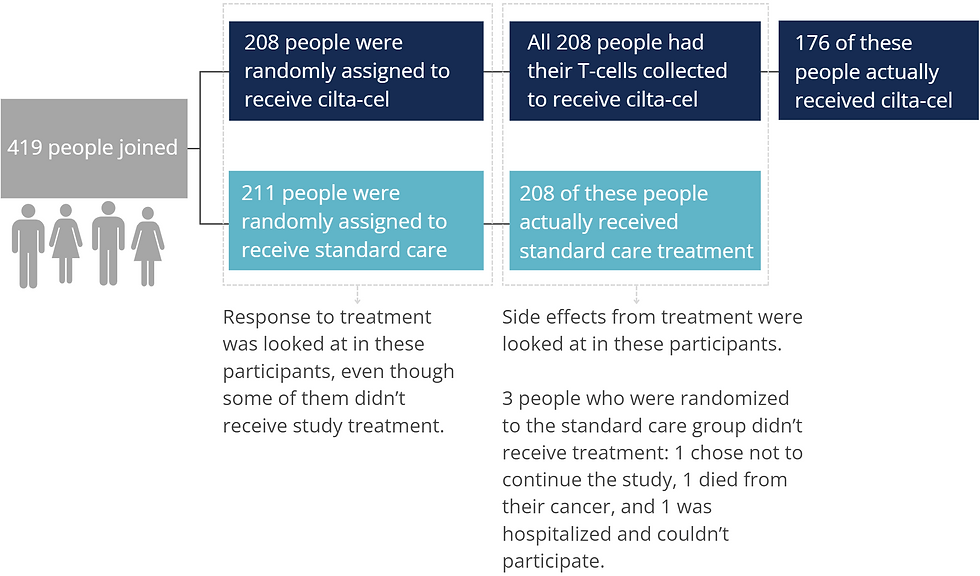
CARTITUDE-4 was carried out at 81 clinics in 16 countries in Europe, North America, Asia, and Australia. Participants enrolled in CARTITUDE-4 sometime between July 2020 and November 2021.

The 419 people enrolled:
had multiple myeloma that was getting worse,
had received either 1, 2, or 3 lines of therapy for their multiple myeloma.
were resistant to lenalidomide, and
had not received CAR-T therapy or any BCMA-directed treatment before.
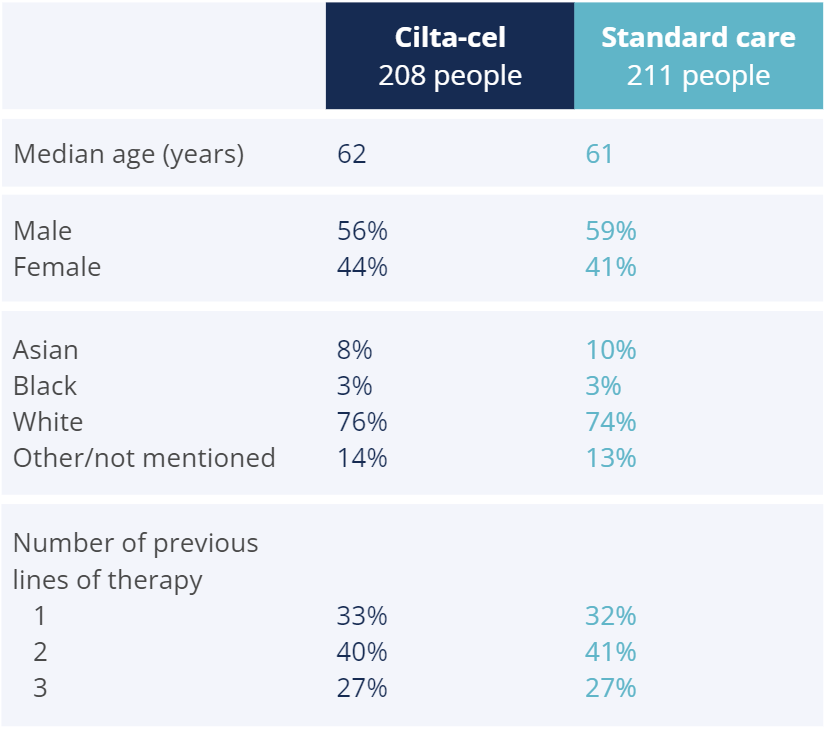
The characteristics of the participants were similar in the 2 treatment groups.
How was the study carried out?
CARTITUDE-4 participants were randomly assigned to a treatment group in a 1:1 ratio. This means that for each person assigned to the cilta-cel group, another person was assigned to the standard care group.
Participants assigned to the standard care group received their doctor’s choice of one of two known effective treatment combinations, either:
pomalidomide, bortezomib, and dexamethasone (PVd) in 21-day cycles, or
daratumumab, pomalidomide, and dexamethasone (DPd) in 28-day cycles.
They received treatment until:
their disease got worse,
they experienced unacceptable side effects from the treatment, or
they chose to leave CARTITUDE-4.
Participants assigned to the cilta-cel group had their T-cells collected using a process called leukapheresis. The T-cells were sent to a manufacturing center to be made into cilta-cel CAR-T cells. Participants received either DPd or PVd to keep their multiple myeloma under control until their CAR-T cells were ready. Starting 5-7 days before receiving cilta-cel, participants underwent lymphodepletion where chemotherapy infusions made space in their immune system for the CAR-T cells. On the day of CAR-T treatment, participants received their cilta-cel CAR-T cells in a single infusion. The median time that participants had to wait from having their T-cells collected until receiving their cilta-cel infusion was 79 days.

All participants were monitored for side effects and had regular tests to check if their cancer was responding to treatment or worsening.
What were the results of the study?
When comparing cilta-cel to standard care, CARTITUDE-4 researchers were most interested in how long participants are able to live after receiving treatment without their cancer worsening.

Approximately 15.9 months after being assigned to a treatment group, the median progression-free survival for the cilta-cel group wasn’t reached yet, meaning that more than half the participants were still living without experiencing disease progression. The median progression-free survival for the standard care group was 11.8 months.
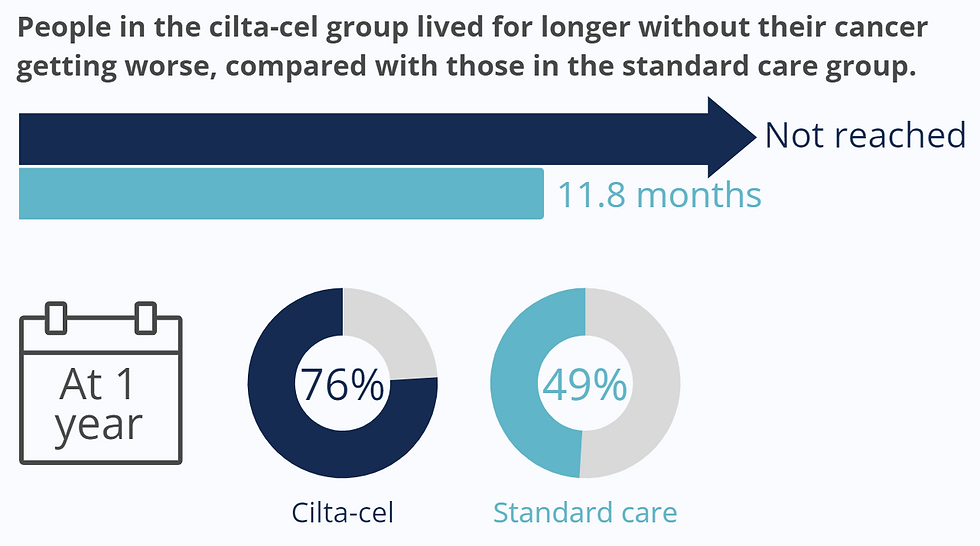
After 1 year, 76% of participants in the cilta-cel group still showed no signs of disease worsening, compared with 49% in the standard care group.
Similar benefit in progression-free survival was seen when CARTITUDE-4 researchers looked at specific groups of participants, including those who:
had high-risk disease features,
had tumors in soft-tissue (outside the bones) at the start of the study,
were resistant to 3 lines of treatment, and those who
had received only 1 line of treatment before the study.
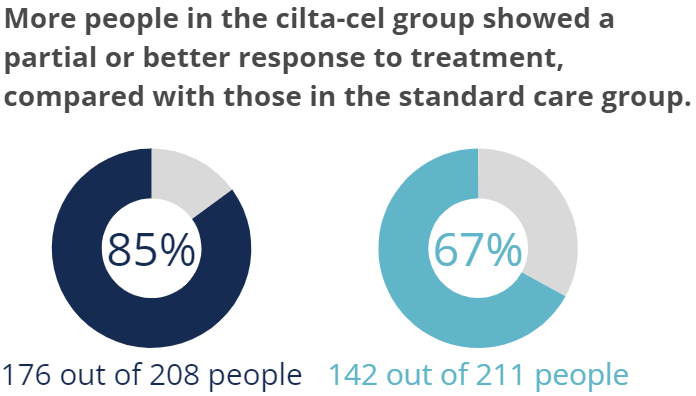
Participants had different types of tests done to check for signs of myeloma in their blood, urine, and bone marrow. Participants who responded to treatment had a decrease in myeloma signs after receiving cilta-cel or while receiving standard care. For people in the cilta-cel group, 85% showed a response to treatment compared with 67% of people in the standard care group.
A “stringent complete response” is the best treatment response possible, as it means no signs of myeloma can be seen in sensitive tests that can detect even small amounts of disease. In comparison, a “complete response” to treatment is when there are no signs of myeloma using standard tests, so some disease may still be present.


Some participants had their bone marrow tested for myeloma cells to check for minimal residual disease (MRD) negativity. MRD negativity is when no cancer cells can be detected in the bone marrow sample. More participants in the cilta-cel group than in the standard care group who had their bone marrow tested showed MRD negativity during the study.
What side effects were seen during the study?
Researchers noted the most commonly experienced side effects from the start of treatment until either 16 weeks after receiving cilta-cel or 30 days after finishing standard care treatment.
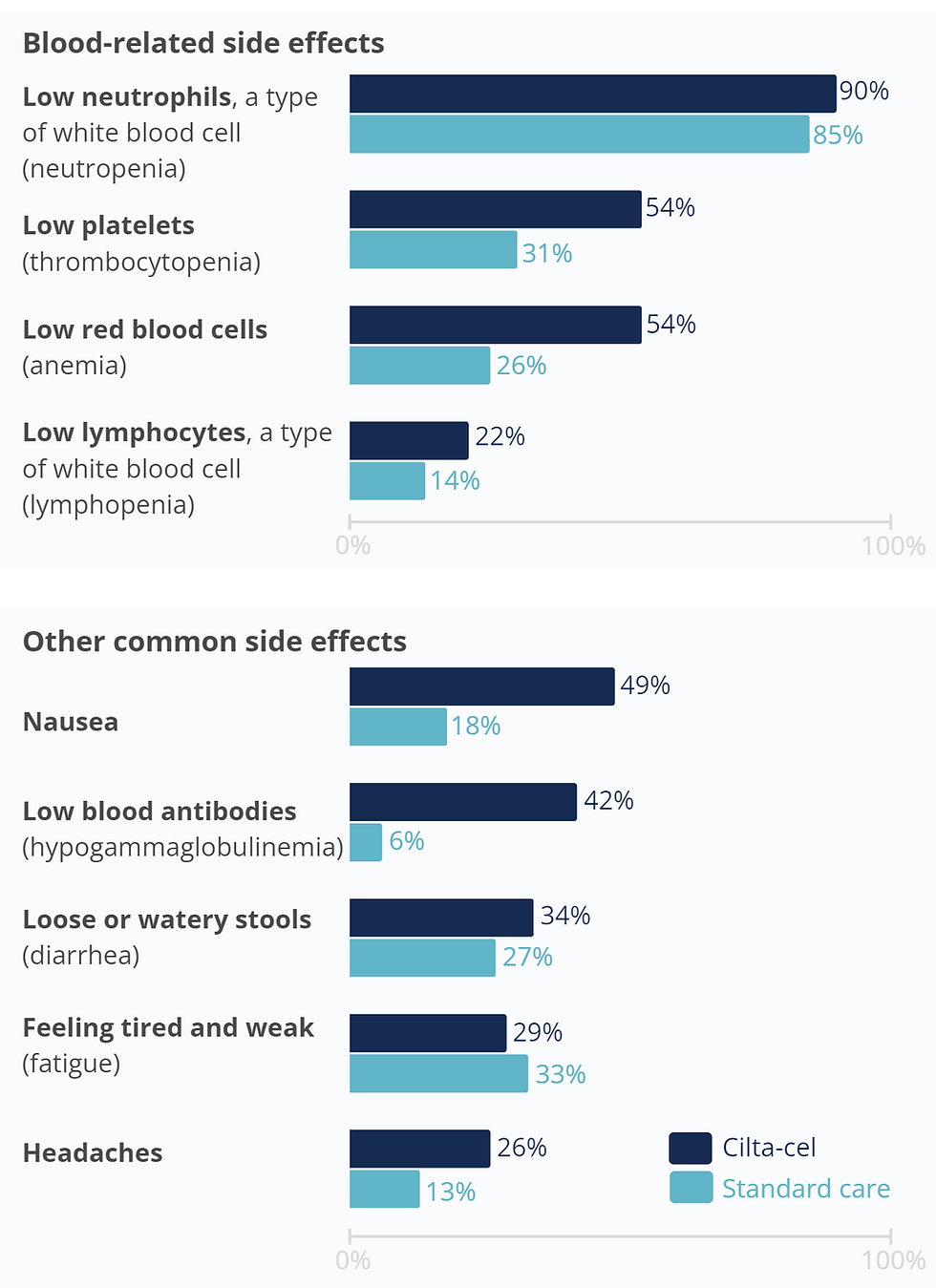
They found that low levels of different types of blood cells were common in both the cilta-cel and standard care groups.
Low levels of blood cells can make people with multiple myeloma more susceptible to infection. This increased risk was seen during
CARTITUDE-4, where 62% of participants in the cilta-cel group and 71% of participants in the standard care group had an infection.
The other most common side effects experienced by people in the cilta-cel group were nausea, low immunoglobulin antibody levels in the blood, diarrhea, tiredness, and headaches.
Participants who received cilta-cel were watched closely for side effects known to occur with CAR-T treatment, including cytokine release syndrome and neurotoxicity. These side effects can potentially be life-threatening, but in CARTITUDE-4, researchers were able to manage all cytokine release syndrome and neurotoxicity events with appropriate medical care.


What do the results of the study mean?
People in CARTITUDE-4 who received cilta-cel lived longer without their multiple myeloma worsening compared with those who received standard care.
People in CARTITUDE-4 who received cilta-cel had a higher overall response to treatment compared with those who received standard care.
Side effects related specifically to CAR-T cell therapy, such as cytokine release syndrome and neurotoxicity, were manageable with appropriate medical care.
Cilta-cel has potential as a treatment option for people with lenalidomide-refractory multiple myeloma who have received at least 1 line of treatment that either didn’t work or stopped working.
Who sponsored the study?
CARTITUDE-4 was sponsored by Janssen Research & Development, LLC.
Where can I find more relevant information?
The original article ‘Cilta-cel or standard care in lenalidomide-refractory multiple myeloma’ was published in the New England Journal of Medicine (San-Miguel J et al, N Engl J Med. 2023;389:335-47).
You can read the full article here: https://doi.org/10.1056/NEJMoa2303379
The CARTITUDE-4 study was registered with ClinicalTrials.gov identifier NCT04181827. Learn more about the study here: https://clinicaltrials.gov/study/NCT04181827
Study start date: 12 June 2020
Estimated study end date: 30 June 2027
A review of important results from clinical trials with cilta-cel, including CARTITUDE-4, was published in Expert Opinion on Biological Therapy (Jagannath et al, Expert Opin Biol Ther. 2024;24(5):339-50).
You can read the full review here: https://doi.org/10.1080/14712598.2024.2352591

